Background
Many patients (pts) with multiple myeloma (MM) will relapse after treatment or become refractory to all therapies currently available. For these pts, the prognosis is poor and there is a clear unmet need to identify agents with novel mechanisms of action. The tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) pathway has a distinct role in the induction of apoptosis in tumor cells, and activating this pathway could represent an attractive therapeutic approach. Early generation TRAIL receptor agonists had minimal efficacy in pts with MM, which may be due to suboptimal TRAIL receptor clustering. Eftozanermin alfa (formerly ABBV-621) is a next-generation TRAIL receptor agonist specifically designed to optimize receptor clustering. The ongoing first-in-human study of eftozanermin alfa (NCT03082209) demonstrated a tolerable safety profile in pts with previously treated solid tumors and hematologic malignancies. Preclinical ex vivo and in vivo models of MM indicate combinatorial activity of eftozanermin alfa with proteasome inhibitors (PIs) resulting in enhanced efficacy. The addition of eftozanermin alfa to the well-established regimen of the PI bortezomib and dexamethasone may improve efficacy outcomes in pts with relapsed/refractory (R/R) MM.
Study Design and Methods
This is a phase 1b, open-label, nonrandomized, multicenter study enrolling pts (≥18 years, Eastern Cooperative Oncology Group score 0-1) with R/R MM. Approximately 40 pts are planned for enrollment. The primary objectives of the study are to determine the recommended phase 2 dose (RP2D) of eftozanermin alfa when given in combination with bortezomib and dexamethasone, and to assess the efficacy of this combination on the basis of overall response rate (ORR). Secondary objectives include assessing the safety and tolerability of eftozanermin alfa with bortezomib and dexamethasone and evaluating the rate of very good partial response (VGPR) or better, the duration of response (DOR), and pharmacokinetics (PK).
Key inclusion criteria are pts with R/R MM who have received at least 3 but no more than 6 prior lines of therapy (including an immunomodulatory agent, a PI, and an anti-CD38 antibody) and have documented disease progression during or after the most recent therapy. Pts must also have measurable disease per standard International Myeloma Working Group (IMWG) criteria at baseline and adequate hematologic, hepatic, and renal function. Pts will be excluded if they have primary refractory disease, received bortezomib in the most recent prior therapy, or have peripheral neuropathy grade ≥2 or grade 1 with pain.
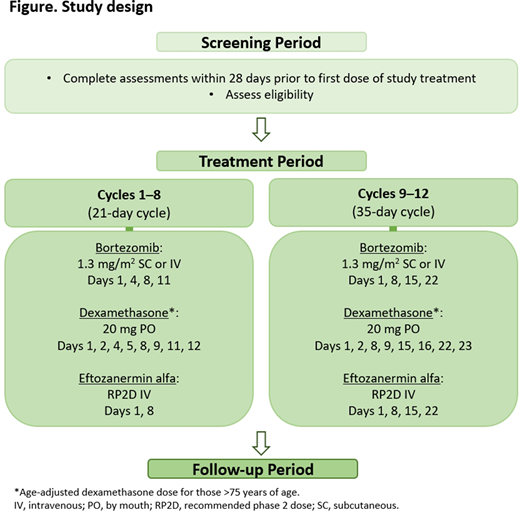
The study consists of a screening period, a treatment period, and a follow-up period; dosing schedule details are presented in Figure. After screening, pts will receive eftozanermin alfa with bortezomib and dexamethasone until unacceptable toxicity, confirmed progressive disease, or other protocol-specified reasons for discontinuation are met. Treatment may continue for maximum 12 cycles. A safety lead-in will be performed to determine the RP2D of eftozanermin alfa when combined with bortezomib and dexamethasone. Dose escalation during the safety lead-in will be guided using a Bayesian optimal interval design with a target toxicity rate of 33%.
The primary efficacy endpoint is the ORR, defined as the proportion of pts with partial response or better per IMWG criteria. Other efficacy endpoints include DOR, VGPR or better rate, and progression-free and overall survival. For all binary endpoints, the estimated proportion and associated 2-sided 90% Clopper-Pearson exact confidence intervals will be provided. The time-to-event endpoints will be summarized using the Kaplan-Meier method. Safety evaluations will include assessment of dose-limiting toxicities during cycle 1 of the safety lead-in, and monitoring of adverse events, vital signs, and clinical laboratory measures throughout the study. Samples for PK analysis, biomarker assessments, and assays for antidrug and neutralizing antidrug antibodies will be obtained at protocol-specified visits. Biomarker analyses may include evaluation of death receptor 4/5 expression, chromosomal abnormalities, and minimal residual disease status. Approximately 20 sites in 6 countries are planned to be involved in the study, which is anticipated to start in September 2020.
Richardson:Celgene/BMS, Oncopeptides, Takeda, Karyopharm: Research Funding. Polepally:AbbVie: Current Employment, Other: may hold stock or stock options.. Motwani:AbbVie: Current Employment, Current equity holder in publicly-traded company. Mu:AbbVie: Current Employment, Other: may hold stock or stock options.. Salman:AbbVie: Current Employment, Other: may hold stock or stock options.. Penugonda:AbbVie: Current Employment, Other: may hold stock or stock options.. Moreau:Novartis: Honoraria; Sanofi: Consultancy, Honoraria; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Honoraria; Amgen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal